Thanks to AI, sciences are transforming with biotech revolution - see 200 million protein DATABANK - DEEP MIND/ALPHAFOLD2
there was 50 year gap between von neumann expectations of ai method and return to generative ai and neuron networks known in his day
its probable that medicine/brugs is at interface betewwen biottech and chemistry- watch crispr gene databank wgih won jennifer doundna nobel prize fir chemistry
here's an axios upd
| Axios Vitals |
| By Tina Reed and Maya Goldman · Dec 11, 2023 |
Happy Monday, Vitals readers. Today's newsletter is 1,086 words or a 4-minute read. |
| |
| |
| 1 big thing: What's next for CRISPR |
 |
|
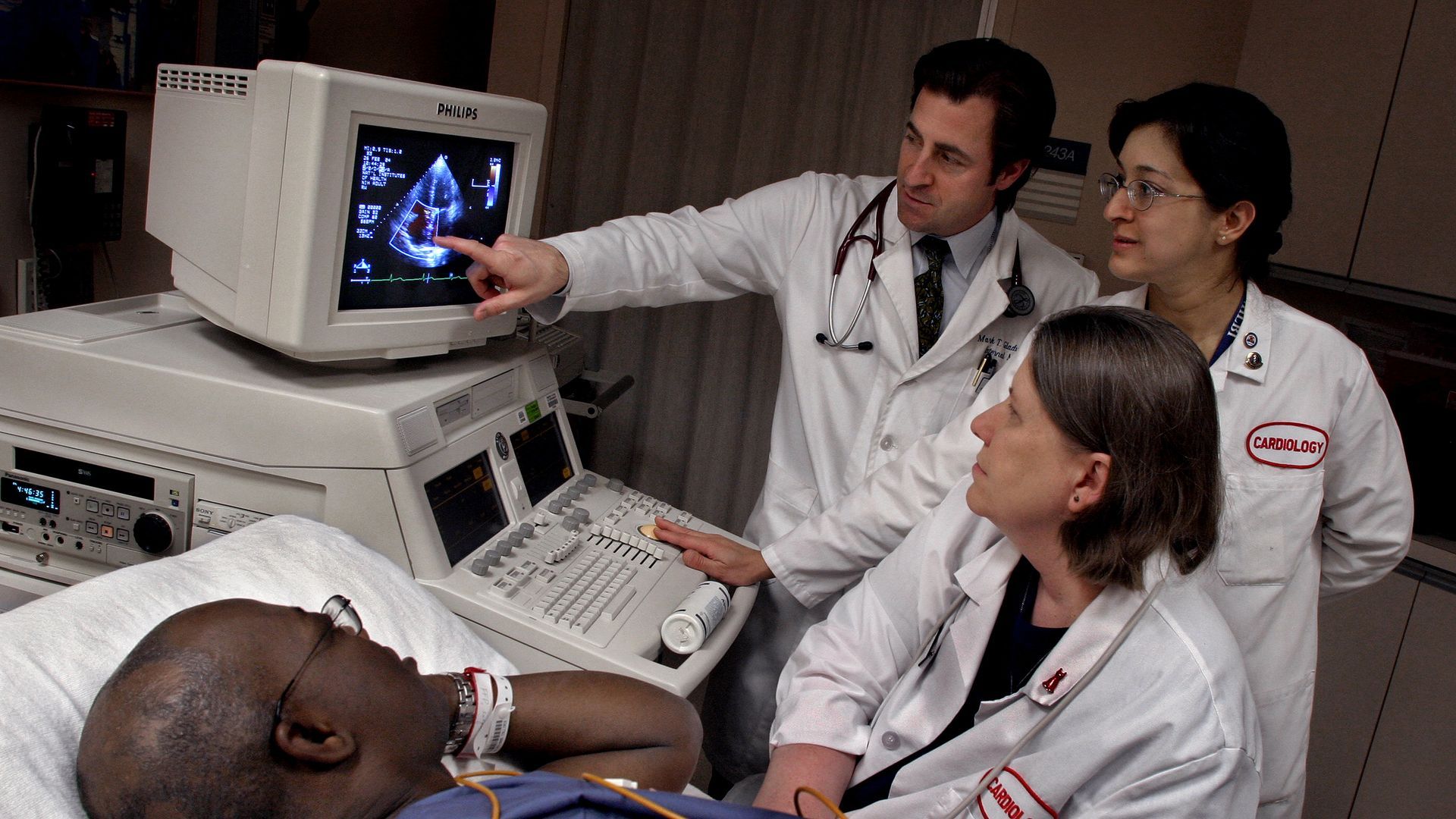
A patient suffering from complications of sickle cell disease gets an echocardiogram. Photo: Michael Williamson/The Washington Post via Getty Images. |
| |
The approval of the first CRISPR-based gene editing treatment to address the excruciating symptoms of sickle cell disease was a landmark moment for the treatment of inherited disorders. - It also raises urgent questions about who may benefit from new cutting-edge treatments costing millions of dollars and what comes next for a technology discovered just over a decade ago, Axios' Adriel Bettelheim writes.
The big picture: Casgevy, from Vertex Pharmaceuticals and CRISPR Therapeutics, is a one-time treatment in which stem cells are harvested from a patient's bone marrow, then edited to produce fetal hemoglobin, which can offset the effects of patients' defective hemoglobin. - It was approved by the FDA on Friday alongside Lyfgenia, another gene therapy treating sickle cell disease, which affects an estimated 100,000 Americans, mostly of African descent, and 20 million people globally.
Yes, but: The treatments aren't for everyone. - Patients first have to undergo extensive chemotherapy to rid their bodies of the defective cells and make way for reengineered ones — a process that could take months and may not be appropriate for older or frail patients.
- Few hospitals can offer Casgevy. Just nine medical centers are now authorized by Vertex to provide the treatment, though the drugmaker will eventually authorize about 50, per New York Times.
- There are other hurdles. For instance, about half of those living with sickle cell are lower-income individuals on Medicaid. The Biden administration launched a recent effort to help ensure this group can access new treatments.
What's next: Like sickle cell, more than 6,000 rare inherited diseases are caused by a single genetic mutation — CRISPR's molecular scissors could hold potential for addressing them, USA Today noted. - Researchers using a newer form of CRISPR last month reported that editing a gene inside the liver can significantly reduce levels of "bad cholesterol" in people who have a genetic condition that causes high cholesterol.
- The study was small but highly anticipated, and researchers believe further study may show it's a powerful tool for reducing heart attacks and strokes, even in people who don't have the genetic condition, per NPR.
- Researchers want to continue to perfect the technology and edit or insert bigger pieces of DNA to make it possible to treat a condition like cystic fibrosis that's caused by many mutations in a certain gene.
Go deeper |

No comments:
Post a Comment